Auer Rods in a Patient with Suspected CMML:
Case Report and the Role of AI-Supported Digital Morphology
About Medilab
Medilab, based in Salzburg, Austria, is a privately owned laboratory offering comprehensive services in clinical chemistry, hematology, microbiology, and molecular diagnostics.
With over 250 skilled employees and processing more than 6,000 patient samples daily, Medilab is recognized as one of Austria’s most advanced diagnostic centers.
As part of its mission to provide highest-quality results with minimal turnaround time, Medilab has embraced next-generation technologies, including the Scopio Full-Field Digital Cell Morphology Platform, which integrates AI-assisted image analysis in hematological diagnostics.
Background and Relevance
The presence of Auer rods in blood or bone marrow smears is a significant morphological marker for myeloid lineage and is most associated with acute myeloid leukemia (AML).
However, these inclusions can also appear in other hematologic malignancies, including chronic myelomonocytic leukemia (CMML), especially during disease progression or transformation.
Despite advancements in molecular and immunophenotypic characterization, morphological findings continue to play a pivotal role in diagnosis, particularly when timely decisions are required in acute settings.
Clinical Presentation
A 44-year-old female patient presented with nonspecific abdominal pain. Initial laboratory evaluation revealed:
- White Blood Cell Count (WBC): 24.8 × 10⁹/L (elevated)
- Absolute Monocyte Count: 14.7 × 10⁹/L
- Red blood cell count and platelets: Within normal limits
- CRP (C-reactive protein): 2.4 mg/dL (mildly elevated)
- LDH (lactate dehydrogenase): 275 U/L (mildly elevated)
Given the abnormal WBC, a peripheral blood smear (PBS) was ordered for manual differential analysis.
Manual Differential Count (Peripheral Blood Smear)
The smear analysis revealed:
- Neutrophils: 5%
- Monocytes: 59%
- Lymphocytes: 28%
- Myelocytes: 1%
- Promonocytes: 1%
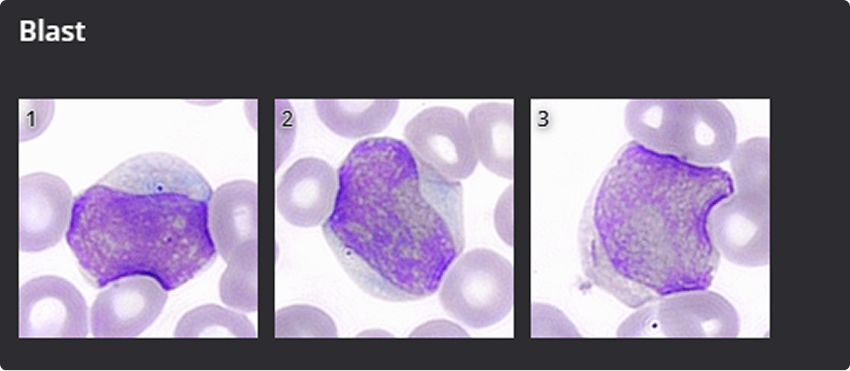
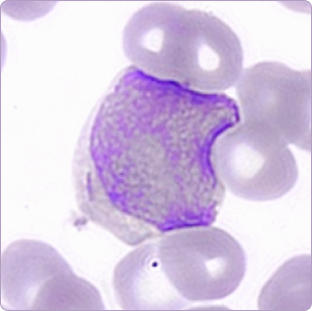
- Blasts: 6%
The findings prompted further investigation due to the marked monocytosis and presence of blasts—hallmarks suggestive of a potential myeloproliferative or myelodysplastic disorder, particularly CMML.
Differential Diagnosis and Clinical Considerations
Monocytosis is defined as an absolute monocyte count greater than 0.8 × 10⁹/L. It is often a nonspecific hematological finding and may occur in a variety of clinical contexts.
Monocytosis is frequently associated with chronic infections, where persistent immune activation leads to an increased production of monocytes as part of the body’s long-term defense mechanism. In addition, it can be observed in connective tissue diseases, such as autoimmune or inflammatory conditions, in which monocytes play a role in mediating chronic inflammation and tissue damage.
Perhaps most importantly, monocytosis may also be a sign of underlying hematologic malignancies, especially chronic myelomonocytic leukemia (CMML), a clonal stem cell disorder characterized by persistent monocytosis and dysplastic changes in the bone marrow.
In any case, the presence of monocytosis should prompt a thorough clinical evaluation to determine its underlying cause, particularly when it is sustained or unexplained.
In this patient, the persistence of high monocyte levels, presence of blasts, and absence of infection signs favored a diagnosis along the myeloproliferative spectrum. While CMML is often diagnosed incidentally, its presentation here was clinically significant.
Importantly, a single elevation of monocytes is not sufficient for diagnosis. Further phenotypic and genetic evidence is essential to rule out reactive causes and confirm malignancy.
Immunophenotyping and Flow Cytometry
In this patient, the persistence of absolute monocytosis, the presence of circulating blasts, and the absence of clinical or laboratory evidence of infection supported a diagnosis within the myeloproliferative–myelodysplastic disease spectrum. Although chronic myelomonocytic leukemia (CMML) is frequently identified incidentally during routine laboratory evaluations, its presentation in this case was clinically overt and diagnostically significant.
It is critical to underscore that a single elevation in monocyte count is insufficient for establishing a diagnosis of CMML. Confirmation requires comprehensive immunophenotypic and molecular characterization to exclude reactive etiologies and substantiate clonal hematopoietic proliferation.
To assess monocyte lineage distribution and exclude reactive monocytosis, flow cytometric immunophenotyping was conducted.
The analysis revealed a predominance of CD14⁺/CD16⁻ classical monocytes exceeding 94% of total monocytes. This immunophenotypic pattern is highly specific for CMML, and when integrated with morphologic findings, it raises substantial concern for progressive or potentially aggressive disease subtypes.
Summary –
Auer Rods and Molecular Profiling in Suspected CMML with AML Transformation Risk
The detection of Auer rods in myeloid precursor cells, while classically associated with acute myeloid leukemia (AML), may also signal impending leukemic transformation in patients with chronic myelomonocytic leukemia (CMML) and is thus considered an adverse prognostic indicator. In the absence of prior hematological records, molecular diagnostics were employed to further refine the diagnostic classification and assess prognostic risk.
Next-Generation Sequencing (NGS) revealed pathogenic mutations in IDH2, NPM1, and NRAS. The presence of IDH2 suggests epigenetic dysregulation; NPM1 is highly indicative of AML transformation; and NRAS correlates with enhanced proliferative signaling and disease progression. This mutational profile supports a diagnosis of high-risk CMML, with a substantial likelihood of evolution into overt AML.
Specialist Summary –
Digital Morphology and AI-Assisted Diagnosis Using the Scopio System
In a complex hematologic case involving suspected chronic myelomonocytic leukemia (CMML) with possible leukemic transformation, conventional microscopic evaluation was enhanced by the integration of the Scopio Full-Field Digital Cell Morphology Platform. A peripheral blood smear was digitally scanned at 100x magnification, enabling high-resolution visualization and AI-assisted pre-classification.
The system confirmed a monocyte fraction of 45.2%, automatically identified circulating blasts, and digitally visualized Auer rods, corroborating manual observations. This digital workflow eliminated diagnostic delays, facilitated real-time expert consultation, and enabled telehematology through full-field, high-resolution image sharing.
Flow cytometric immunophenotyping further verified a monocytic immunoprofile (CD14⁺/CD16⁻ >94%), supporting a clonal etiology. Complementary next-generation sequencing (NGS) revealed pathogenic mutations in IDH2, NPM1, and NRAS, indicative of epigenetic dysregulation, high-risk AML transformation, and proliferative disease progression, respectively.
This case highlights the value of a multimodal diagnostic strategy that integrates traditional morphology, digital AI-enhanced imaging, immunophenotyping, and molecular genetics. The identification of Auer rods—both manually and digitally—served as a pivotal morphologic marker. The Scopio platform significantly contributed to diagnostic accuracy, efficiency, and confidence, illustrating its role as a critical tool in next-generation hematopathology and reflecting the broader digital transformation of laboratory medicine.
